
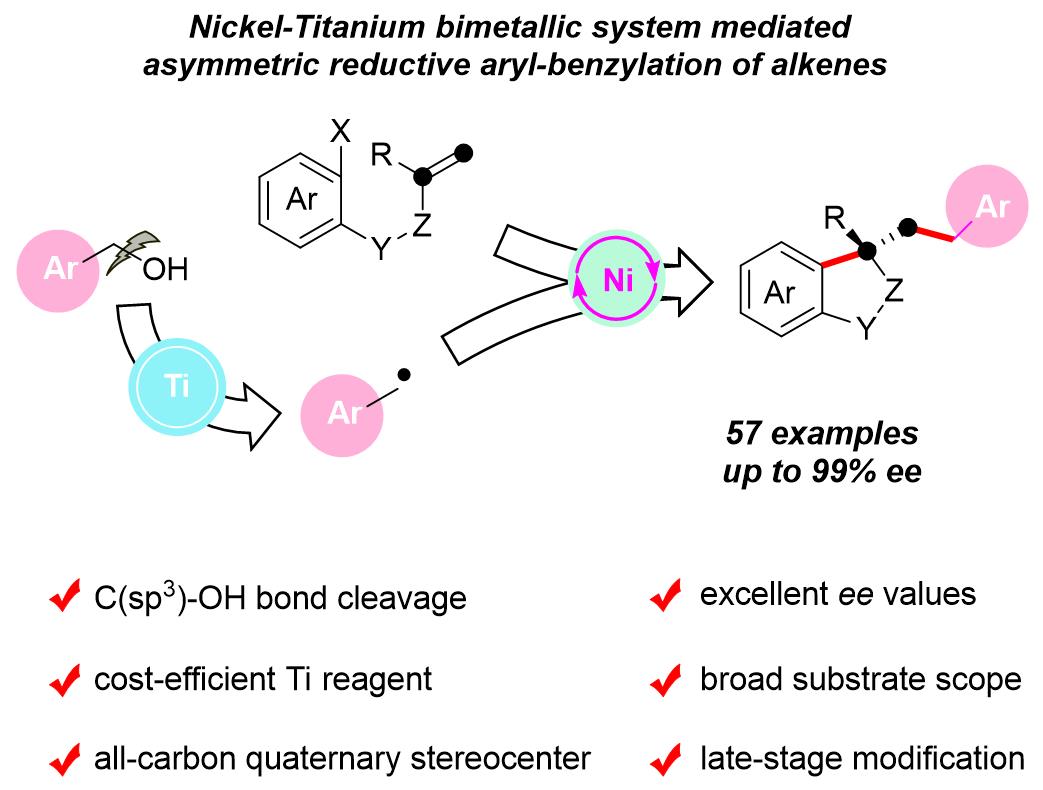
Prof. WANG Xiaoming's group at the Shanghai Institute of Organic Chemistry of the Chinese Academy of Sciences has demonstrated the first nickel-titanium bimetallic system-catalyzed asymmetric reductive aryl-benzylation of alkenes using benzyl alcohols, which afforded a series of biologically important benzene-fused cyclic compounds in good yields with high enantiomeric excess (ee) values.
Difunctionalization of alkenes can rapidly construct complex molecules, in particular, Ni-catalyzed asymmetric reductive dicarbofunctionalization of tethered alkenes is one of the most effective methods for the rapid assembly of various chiral cyclic frameworks. Since alcohols are ubiquitous in nature and medicine, the direct use of alcohols as coupling partners via homolytic C-OH bond cleavage remains a formidable challenge with great opportunities to achieve new and useful transformations.
In addition, low-valent titanium complexes have been considered particularly effective for radical deoxygenation of free alcohols due to their strong oxophilicity, high reduction potential, and cost-efficiency, which plays an important role in the direct deoxygenation of alcohols.
In this context, the combination of inexpensive Ti reagent for direct activation of alcohols with Ni-catalyzed asymmetric reductive difunctionalization of tethered alkenes may offer great opportunities for the synthesis of useful chiral building blocks using free alcohols.
In this study, using the radicals generated in situ by titanium-mediated homolytic C-O bond cleavage of benzyl alcohols as the key species, the nickel-catalyzed transformation proceeds under mild reaction conditions, with a wide substrate range and high functional group tolerance.
It provides easy access to various chiral benzene-fused cyclic compounds, including oxindoles, dihydrobenzofurans, tetralins, indane, and isochroman bearing an all-carbon quaternary center with enantioselectivities of up to greater than 99% ee in a single step, according to the researchers.
The synthetic utility of the protocol has been demonstrated in the late-stage modification of pharmaceuticals and the streamlined synthesis of the structural scaffolds of natural products.
Preliminary mechanistic studies suggest that the reaction is most likely to proceed via a Ti-mediated direct homolysis of the alcoholic C-O bond, followed by the interception of the benzyl radical with σ-alkyl-Ni(II)X species in a Ni-catalyzed asymmetric cross-coupling process.
This work was published on Cell Reports Physical Science and it was supported by the China Postdoctoral Science Foundation, the National Natural Science Foundation of China, the University of the Chinese Academy of Sciences, among others.

86-10-68597521 (day)
86-10-68597289 (night)

86-10-68511095 (day)
86-10-68512458 (night)

cas_en@cas.cn

52 Sanlihe Rd., Xicheng District,
Beijing, China (100864)

